I have had a feeding tube since January 2012 because, thanks to ALS, I can not swallow food or liquids. I have been nourishing myself since I got the tube. The tube is called a
PEG tube. I have a hole in my stomach to accommodate it.
A Clogged Feeding Tube
On Saturday, December 8, 2012 my tube became clogged. I couldn’t get anything through it. With the help of our hospice
team, I ended up in the emergency room, where a knowledgeable and very kind doctor determined that he couldn’t replace the tube, but he could unclog it. He advised that I promptly contact the doctor who had put in the tube I had to find out about getting it replaced.
On Tuesday, December 11 (2 days after the ACLU awards ceremony (the speech I gave at that event is the last blog I posted before this one), I was at the hospital to have my tube replaced by the same gastrointestinal surgeon who placed the original tube. Unlike the original tube, the replacement did not require me to be under general (or any)
anesthetic. That was good: I didn’t want general anesthetic because I didn’t want to risk being permanently on a ventilator after surgery, or to expose myself to the risk that general anesthetic would hasten the progress of my ALS.
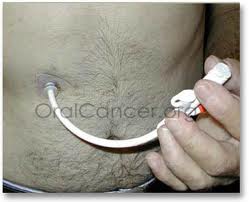
The replacement tube can easily be put in (or removed) by pushing it (or pulling it) through the hole in my stomach. It has a balloon that holds it in place in the stomach.
That’s the good news. Also, the replacement tube was not clogged. Also good news. The rest of the news about this tube is not good.
A Little Background About Nourishing with a PEG Tube
The nourishment process involves several steps. All of them involve putting the tip of a fluid-filled bolus [think of a cartoon-sized syringe] into the opening of the tube that’s called
a port. These are the steps:
1) Close the clamp on the tube so that stuff does not come out when the port is opened to insert the tip of the bolus.
2) The bolus is filled with water that will be flushed through the tube. The port is opened, and the tip of the bolus is inserted. The clamp is opened so the liquid can pass through. When the bolus is empty, the clamp is closed before the bolus tip is removed.
3) The bolus is filled with liquid nourishment that will be pushed slowly through the tube. This is repeated until multiple times until the amount of nourishment for the meal is consumed. The clamp is closed and opened and closed again with each bolus.
4) The bolus is filled two more times with water that is flushed throughout the tube. The clamp is closed and opened and closed again with each bolus.
Medications are also dissolved in water and put through tube, with a water flush preceding and following the meds.
The Replacement Tube
The replacement tube came with no clamp! As you can see from the description above, this lack of a clamp is a big problem. If there is no clamp, there is no way to keep liquid from coming out through the port when the bolus is removed and before the port can be closed.
There’s a second problem: the port for receiving the bolus is very shallow, so the bolus can and does slip out, spilling liquid. The first time we used the new tube, I needed a shower afterwards.
With the help of the surgeon’s nurse and our hospice nurse, we obtained a surgical clamp to solve the clamp problem, but this requires two people when the tube is in use: one to hold the bolus securely in the port, and another to open and close the clamp.
The manufacturer of this tube, called an EndoVive, is Boston Scientific. I contacted the Boston Scientific rep for this area by email. In that message, I laid out the design problems
with the replacement tube.
I closed my email with the following paragraph:
“ALS patients have many problems. Boston Scientific should not add to those problems with sloppy design. My previous tube had a clamp on it that I could open and close easily with my thumb. It’s not rocket science. Boston Scientific should be able to quickly add such a clamp to the EndoVive product. While you are doing that, I believe it should be possible to modify the valve to accept a standard-sized 60 ml bolus.”
The Boston Scientific rep passed my email on to the person at the company responsible for managing this product. He agreed that the company should not be making products that make my day-to-day life (or presumably anyone’s day-to-day life) more difficult. In a telephone conversation with my partner Susie, he told her that he regretted that no one at the company thought of these issues. He also said there was not quick fix, since any changes to the design would have to approved by the FDA.
In the meantime, our contact at the California Home Medical Equipment, which supplies my nourishment and necessary accoutrements, began to pursue solutions. He found a  clamp that could be added to after the tube was in place, and an extension tube with a much deeper port. The only remaining challenge at the moment that is keeping me from being able to nourish myself is that my weakened hands can not close the new clamp. So, for that, I need help.
clamp that could be added to after the tube was in place, and an extension tube with a much deeper port. The only remaining challenge at the moment that is keeping me from being able to nourish myself is that my weakened hands can not close the new clamp. So, for that, I need help.
Of course, at some point, I won’t be able to nourish myself as the ALS progresses. But the tools needed by ALS patients and others with disabilities should be designed to allow them to function independently as long as possible. This feeding tube by Boston Scientific does the opposite.
In looking on line for other replacement PEG tubes that are balloon anchored (and therefore can be inserted without general anesthetic), I found several others, none of which come with a clamp. I could not tell the depth of the ports on those tubes from the information I found on line.
I am frankly appalled that companies and the FDA seem to think that these types of replacement PEG tubes are appropriate. It seems to me that there were never tested on patients or with caregivers who might know what the practical issues are. After all, I can’t possibly be the first person with PEG tube to have this problem. Why was this design approved in the first place?
A Call to Action
If you agree, send an email to the FDA Center for Devices at dsmica@fda.hhs.gov. Demand that the FDA consider patient needs in the device approval process.
© Barbara A. Brenner 2013





Is the hospital that placed this tube still using them? It seems to me that their purchasing folks (and their peers at other healthcare delivery organizations) need to make sure that they aren’t buying things that don’t work for patients. My wild guess is that they can reject these with their purchasing power in the marketplace more quickly than the FDA. A thought fwiw.
A good thought, if only it worked that way. First of all, there is very little that works (see the post and other comments to this blog. Second, this is a Sutter Health Care facility that pretty much dominates the health care market in Calfifornia (what they don’t control is controlled by what used to be called Catholic Charities –they recently renamed themselves to try to fool people about who they are.) Sutter is about making money, not making sure things work for patients.
Did your doctor share with you other options available other than the bolus delivery system???
I know about the drip delivery system, otherwise, no.
Oh Barbara, you have suffered. When i went to reorder a clamp, it came from tool supply company! Also i have a bolus tube holder that has been a lifesaver. Check out the website http://www.feedingtubeholder.com/. Thank you for all you do.
Hi Deon my husband had the same problem, fortunately we changed to a Mickey Peg Tube, they are amazing they clip off right at the tummy and you can wash the feed tube after use as well the mickey stays in place with eh inflated balloon in the tummy can be changed in under 20 mins in your own home by yourself or family menber as well, we keep a spare in the house and you get extra attachments as well. They are from a company called Peri Hill. Will not change from this product. Bernice and Deon South Africa
Barbara,
Barbara,
I truly admire your courage and your spirit.
As you suggested I wrote to the FDA.
Some of us have had good results with a US-based stem cell treatment plan.
https://sites.google.com/site/williamsstemcellals/edit
We (ALSTUN)
http://treatalsnow.org/
are also stepping up our efforts thanks to a now retired attorney and Former HIV activist with a capital A.
Thank you so much for helping us get started.
Best to you,
Ed
I was happy to send a letter to the FDA. You will always have a place in my heart.
I have been a home care nurse forever…but only recently came across the EndoVive tubes. I agree all extension tubes should come with clamps. After working with the EndoVive Low Profile Button for a month now, I still like a Mickey much better.
When I called Boston Scientific for some info, they said they don’t offer clinical support. My patient, and his nurses/family, didn’t even get a decent instruction booklet, like the Mickey offers…I have heard it is available, will have to make some more phone calls. One would think it would be available on line, but all the company info is geared towards sales sales sales.
I will gladly send a letter to the FDA. So much money (tax dollars) is wasted on medical equipment sold/ordered that is not practical for the person it is ordered for.
Carry on!
Nancy Nurse